The Turkish parents of a young boy diagnosed with a life-threatening genetic disorder have hailed the hospital that offered him a second chance in life, thanks to donors who raised $2 million to pay for his care.
In November regulators in the UAE fast-tracked a novel gene therapy to treat the debilitating symptoms of spinal muscle atrophy (SMA). The severe genetic condition is characterised by degeneration of motor neurons, resulting in progressive muscle weakness, impaired breathing, and loss of motor function.
Successful trials of the therapy demonstrated sustained improvements in patients' motor abilities, prompting UAE regulators to approve the single-dose drug Itvisma, made by the Swiss pharmaceutical company Novartis.
One of the first to benefit was Turkish toddler Hulusi Sert, four, who was administered the treatment last month in Dubai, after his parents Mustafa, 32, and Remziye Sert, 30, raised $2 million from community donors in Turkey. It took the family 29 months to raise the money.
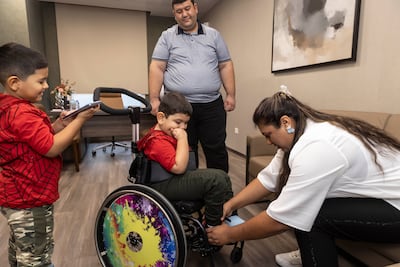
“When Hulusi was three or four months old, he started getting shaking hands and legs, so we knew something was wrong with him,” Mr Sert, who works in a hospital in Tekirdag delivering food for recovering patients, told The National. “We took him to see the doctors, but they were saying there was nothing wrong and that it was just a psychological issue.
“We finally approached one neurologist who directly asked if the hospital had done a test for SMA, and they said no because no one had asked for one. They did the test and when they got the result, he was diagnosed with SMA. He was 14 months old then.”
Transformative results
Gene-based therapies have transformed outcomes for affected children, particularly when delivered early and within specialised, multidisciplinary care environments.
The biggest challenge is paying for the treatments, which are among the most expensive in the world. Typically only the highest insurance plans cover the costs, leaving families to find other sources of fundraising or charity donors.
“It was really difficult to fund this in Turkey, as it was such a large amount,” Ms Sert told The National. “Because it was taking so long, Hulusi started losing movement in his legs. He stopped walking, and couldn’t use his legs so we had to get him into a wheelchair.
“It was very difficult for us, but thankfully through community donations we were able to raise enough money for this treatment in Dubai. We needed authorisation from the government to start fundraising, otherwise there was nothing from their side other than to transfer the funds directly to the hospital.
“From the beginning, we didn't know anything about SMA, or understand anything about how severe it is. After having a child diagnosed with this disease, we started learning each part of this disease pretty quickly.”
Hulusi is likely to remain in hospital in Dubai for another three or four months, alongside his parents and his older brother Ramazan, who is six. His progress will be closely monitored by Dr Vivek Mundada, a consultant paediatric neurologist at Medcare, who said regular tests will assess improvement in his muscle growth and motor skills.
“Significant funding for this kind of therapy can take years – young children do not always have that time,” said Dr Mundala. “Turkish patients tend to raise the money faster because they have a very structured programme. SMA is a very complex disease. It's just not about muscle wastage, because there's secondary complications through respiratory and cardiac systems, even to swallow.
"I don't want to give parents false hope. It's not about reversing the condition and giving some magic so their child is going to run a marathon. We have to give a realistic expectation of what they want to achieve and what this drug is going to do. It's high-stakes due to the costs.”
Walking unaided
While the full extent of recovery Hulusi will experience is unknown, he is expected to soon be able to walk unaided and grow up as a normal young boy.
SMA is a relatively common genetic disorder, affecting between one in 6,000 to one in 11,000 live births. It is a significant cause of infant death, and it is estimated that around one in 40 people are healthy carriers of the altered gene, which can be passed on to children.
Itvisma can be administered to children over two years old or who weigh more than 21kg. Doctors also claim the treatment can be given to adults with a late diagnosis of SMA, in a milder form.
The UAE is quickly becoming a go-to regional destination for the last treatments and therapies. Fakeeh University Hospital recently became one of the first in the region to administer Duvyzat (givinostat), an advanced therapy used in the management of Duchenne muscular dystrophy (DMD).
DMD is another rare, inherited neuromuscular disorder that primarily affects young boys, leading to progressive muscle weakness, loss of mobility, and serious cardiac and respiratory complications over time. Early and advanced intervention is critical to slowing disease progression and improving both survival and quality of life.
Over the past 12 months, Fakeeh University Hospital has provided gene therapy-based treatments to children from multiple countries, with consistently positive clinical outcomes.
“The outcomes we are seeing, and the trust placed in us by families travelling from abroad, reinforce our commitment to continue expanding these services,” said Dr Arif Khan. “Gene therapy is rapidly extending beyond neurology into areas such as haematology and inherited metabolic diseases.
“Our long-term vision is to establish a comprehensive centre of excellence that supports the expanding role of gene therapy across specialities, positioning Dubai as a regional and international hub for advanced genomic medicine.”


