Parents are banking stem cells from their children’s milk teeth in the hope they can be used in the “next frontier” for treating diabetes.
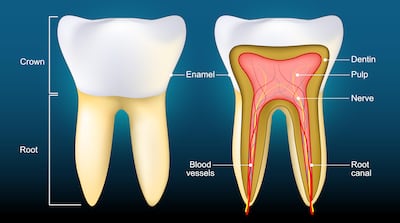
Some companies even claim "dental pulp" is already being used in treatments for debilitating and potentially life-threatening conditions, an investigation found. Dental pulp is the soft inner tissue found near the centre of a tooth that contains nerves, blood vessels and connective tissue.
The investigation by the BMJ healthcare professionals' publication warned that companies may be misleading parents with “outrageous claims” about how teeth can be used, saying suggestions about their future medical value are “unproven and potentially misleading”.
They also highlight current research using stem cells in multiple sclerosis, Parkinson’s disease and heart attacks.
Experts were alarmed by claims they could already treat autism and diabetes, which will now be reviewed by the UK’s Advertising Standards Agency.
Tooth stem cell banking costs around £1,900 ($2,560), with an additional annual storage fee of £95. Customers sign an agreement before being sent a collection kit when a child’s tooth becomes wobbly. Lost teeth are then sent to a laboratory where the dental pulp is digested, stem cells cultured until a sufficient number are present, harvested then stored in liquid nitrogen.
Three companies in the UK offering tooth stem cell banking – Future Health Biobank, BioEden and Stem Protect − all operate through one laboratory.
Stem cells were first isolated from teeth in 2000, and less than a decade later companies began to offer tooth stem cell banking. These stem cells are “especially valuable because they are younger and healthier", Future Health Biobank says.
Scientists have debated whether stem cells derived from dental pulp are comparable with those derived from bone marrow.
Future Health Biobank says on its website that it has released 26 tooth stem cell samples for treatment − including for autism, Type 1 diabetes and knee cartilage regeneration − all to private clinics in North America. It said it would review how information on its site is presented to ensure “readers can clearly distinguish between client experiences and formally published clinical outcomes”.
The company also says it has a “robust, ongoing, storage stability validation programme” with quality control testing “to ensure that there is no deterioration in the integrity, viability or future potential of biological samples”.
The BioEden website says stem cell therapy has been described as the “next frontier” for treating Type 1 and Type 2 diabetes. It states it has “already witnessed the remarkable evidence of these ongoing developments” among its own customers.
One 28-year-old treated with dental cells has reported “decreased swelling, an improvement in energy levels and a reduction in their insulin application", the website states.
“Another member, who’s halfway through their treatment, has had similar results along with improvements in their liver function, and is to begin their second course later this year,” it added. The "best stem cells are young stem cells", which is why it is “advisable” to bank at the youngest age possible, it claims.
Stem Protect refers to cleft palate repair, sickle cell disease, HIV/AIDS, severe combined immunodeficiency, and knee cartilage repair under a section of its website with the heading: "What treatments are tooth stem cells used for?"
But several experts are concerned about the claims being made.
Jill Shepherd, senior lecturer in stem cell biology at the University of Kent, said companies are selling the “potential” for something that is not yet borne out by the science. “There is a lack of evidence and a paucity of research using dental pulp stem cells to treat patients.”
Sufyan Hussain, a clinician at King’s College London and an investigator on the UK arm of a global clinical trial evaluating stem cell therapy for Type 1 diabetes, said work on dental pulp stem cells was at a very early pre-clinical stage.
“The stem cells being studied in clinical settings are predominantly from either donated embryos or sources readily available from adults," he said. "To my knowledge there is currently no active human clinical research using stem cells derived from baby teeth to treat diabetes. At present, there isn't a definitive answer regarding the optimal source of stem cells for future diabetes therapies.”
Diabetes UK said more research was needed before it recommended people engage with commercial companies who are banking stem cells.
Tim Nicholls, assistant director of policy, research and strategy at the National Autistic Society in the UK, said it was “outrageous” that tooth stem cell procedures are being advertised to parents with the false claim of treating autism.
“Autism is not a disease or illness, it cannot be treated and there is no cure,” he said. “It is dangerous and morally bankrupt to target potentially vulnerable people with expensive procedures that could, in fact, cause harm.”
Experts are also concerned about the lack of independent information on tooth stem cell banking to help consumers make a fully informed choice, and say more oversight is needed of the information being used to promote the practice.
Ms Shepherd also believes parents should be given more information on what type of tests are done to validate that stem cells are present in the stored samples, how they were collected and how long they can be viably stored.
She also points out that the phrase “stem cell” is being used as a catch-all. Websites list many pages of research, but these are unlikely to be from dental derived stem cells.
“It can be misleading," she added. “Such decisions can be emotive for parents. But there isn’t the information out there to inform them, and the regulators should take an active role.”
The BMJ findings were sent to the Advertising Standards Agency, which says it will review them.