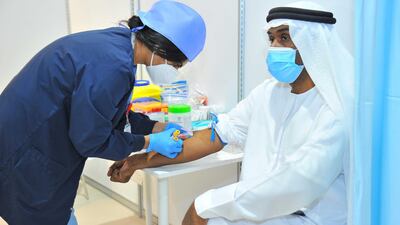
More than 10,000 people have volunteered to be part of Abu Dhabi's Phase 3 clinic trials for a vaccine to fight Covid-19.
The volunteers represent 20 different nationalities taking part in the 42-day trial, Abu Dhabi's health department said.
Dr Jamal Al Kaabi, the acting undersecretary of the department said that the large number of participants "will help demonstrate that the trials have a high chance of efficacy and show the vaccine’s effects across a wide range of demographics".
Abu Dhabi and Al Ain are home to the world’s first World Health Organisation-enlisted Phase-III trial, which is the final step before a vaccine is approved for use among the public.
The vaccine being trialled in Abu Dhabi is 'inactivated'. It is one type of vaccine designed to protect people against the disease and uses a "killed" version of the virus that causes the illness.
Phase 3 trials typically evaluate the effectiveness of a drug on a large population sample and scientists hope it will help identify specific antibodies that provide immunity. More than 20 vaccines are in clinical trials globally, while another 140 are in early development globally against the disease. Only three are in phase 3 trials.
Volunteers are still being accepted for the UAE trial, which takes place at five sites in the capital and Al Ain. About 5,000 volunteers will be involved in the study at first, the health department said.
"The campaign not only helps in combating Covid-19 but will provide a highly effective approach to measuring the vaccine’s effects," said Dr Nawal Al Kaabi, the chair of the National Clinical Committee for Covid-19 and the executive medical director at Sheikh Khalifa Medical City.
The UAE trial is a partnership between Chinese vaccine manufacturer Sinopharm, Abu Dhabi-based artificial intelligence company Group 42 and Abu Dhabi Health Services Company, Seha.
It is being supervised by Abu Dhabi's health department in accordance with guidelines outlined by the World Health Organisation and the Food and Drug Administration of the United States.
“A team of specialist medical practitioners from Seha ... will be managing and involved in conducting the clinical trials. All trials will be administered at dedicated centres equipped to accommodate the volunteers, which include citizens, residents of all nationalities and both sexes,” Dr Al Kaabi said.
Trials for Phase-1 and 2 took place in China.
Up to 15,000 volunteers are wanted and they must be in good health and between the ages of 18 to 60. Sheikh Abdullah bin Mohammed Al Hamed, chairman of the Department of Health Abu Dhabi, was the first volunteer to receive the jab.
Hamad Al Balushi, an Emirati taking part in the trial and one of the first to be given the vaccine hopes that the study will be of benefit "to both the UAE and global communities", a statement from the health department on Friday said.
Another volunteer from Oman spoke of his pride to be one of the first volunteers "in this historic trial and invite all my brothers and sisters to join me".
Volunteers must visit testing centres a minimum of 17 times over about 42 days.
They cannot travel abroad during this time. After the trial, they will have regular phone consultations for up to six months.
People can register on the government website 4humanity.ae or call 02 819 1111.